anti-sars-cov-2 neutralizing antibody elisa kit|SARS : supplier The cPass SARS-CoV-2 Neutralization Antibody Detection is a blocking Kit ELISA test that detects functional immunoglobulins neutralizingthe interaction . anti-SARS-CoV-2 neutralizing antibodies .
The out-of-autoclave (OoA) process manufactures composites by applying vacuum, pressure, and heat outside of the autoclave. This review discusses the common out-of-autoclave processes for various applications.
{plog:ftitle_list}
Sitemap - Modi Enterprises (India)
The Human SARS-CoV-2 Neutralizing Ab ELISA (enzyme-linked immunosorbent assay) is designed to measure the neutralizing portion of anti-SARS-CoV-2 antibodies. A receptor .The SARS-CoV-2 Neutralizing Ab ELISA kit is designed to measure the neutralizing portion of anti-SARS-CoV-2 antibodies. A receptor binding domain (RBD) protein is pre-coated in the wells of the supplied microplate.Cayman’s SARS-CoV-2 Neutralizing Antibody Detection ELISA Kit is a competitive assay that can be used for qualitative and/or semi-quantitative measurement of neutralizing antibodies in human plasma and serum. Commercially available SARS-CoV-2 serum-based antibody detection ELISA kits from Gold Standard Diagnostics (GSD) and EuroImmun (EI), which detect the nucleocapsid (N) and spike (S) SARS-CoV-2 .
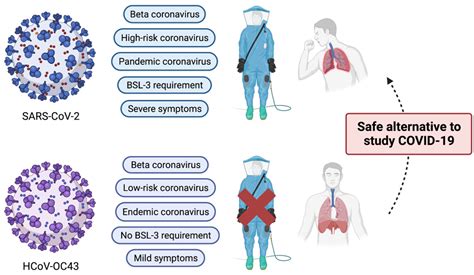
SARS-CoV-2 Neutralizing Antibody Assay Kit (DEIASL055), manufactured by Creative Diagnostics with high sensitivity and reproducibility, providing efficient and accurate result for lab research. . Human Anti-SARS-CoV2(N) IgM ELISA Kit. SARS-CoV-2 Nucleocapsid IgG ELISA Kit. SARS-CoV-2 Nucleocapsid IgM ELISA Kit. SARS-CoV-2 IgG/IgM Rapid Test . The cPass SARS-CoV-2 Neutralization Antibody Detection is a blocking Kit ELISA test that detects functional immunoglobulins neutralizingthe interaction . anti-SARS-CoV-2 neutralizing antibodies . In SARS-COV-2, infection and immunization are associated with developing variable levels of antibodies with neutralizing activity; thus, evaluating the neutralizing capacity of anti-SARS-CoV-2 antibodies is important because they represent real protective immunity. 3 While the plaque reduction neutralization test (PRNT) is commonly considered the gold standard for .COVN-E SCoV-2 Detect™ Neutralizing Ab ELISA (EUA) Insert Part No. 900278- 00 Effective Date: 10/22/2021 Page 3 of 14 WARNINGS AND PRECAUTIONS • For use Under Emergency Use Authorization
Several potent SARS-CoV-2 antibodies have shown degrees of success in preclinical or clinical trials, and the US Food and Drug Administration has issued emergency use authorization for two neutralizing antibody cocktails.Nevertheless, antibody therapy for SARS-CoV-2 still faces potential challenges, including emerging viral variants of concern .The SARS-CoV-2 Neutralizing Antibody ELISA Kit is a competitive Enzyme‑Linked Immunosorbent Assay (ELISA) designed to detect the level of SARS-CoV-2 Neutralizing Antibody in serum and plasma. The 96-well plate is coated with a SARS-CoV-2 Receptor Binding Domain (RBD) antigen. Samples with neutralizing antibodies compete withAnti-SARS-CoV-2 ELISA (IgG) 05/04/2020: IgG, ELISA . ThermoGenesis SARS-CoV-2 IgM/IgG Antibody Test Kit: 02/14/2022: . SCoV-2 Detect Neutralizing Ab ELISACOVID-19 Neutralizing Antibody ELISA Kit is an indirect enzyme-linked immunoassay (ELISA) for semi-quantitative determination of COVID-19 neutralizing antibody.SARS-CoV-2,Coronavirus,COVID-19,COVID19,Wuhan virus,Wuhanvirus COVID-19 Neutralizing Antibody ELISA Kit, KA6111 is ready to ship. . HRP Anti-Tag Antibody 7. Blocking Buffer Concentrate .
SARS
The Human SARS-CoV-2 Neutralizing Ab ELISA (enzyme-linked immunosorbent assay) is designed to measure the neutralizing portion of anti-SARS-CoV-2 antibodies. A receptor binding domain (RBD) protein is pre-coated in the wells of the supplied microplate.
Threefold serially diluted monoclonal antibodies including anti-SARS-CoV-2 Neutralizing human IgG1 antibody from Acro Biosystems, NAb#1 (Fig. 4c, d), GenScript clone ID 6D11F2, NAb#2 (Fig. 4d) and . A monoclonal antibody (mAb), 20D8, was identified as the detection antibody based on its high RBD binding activity (EC50 = 8.4 ng/mL), broad-spectrum anti-variant neutralizing activity (EC50: 2.7−9.8 ng/mL for pseudovirus and EC50: 9.6−127 ng/mL for authentic virus), good in vivo protection, and a recognized linear RBD epitope (369−379 aa).
To support COVID-19 research, Sino Biological has developed a large collection of antibodies against different antigens of SARS-CoV-2. These antibodies can be used to detect and/or neutralize the virus in a variety of assays including ELISA, western blot, Immunofluorescent staining, Immunohistochemistry, SPR (Biacore and Octet), pseudovirus infection, and . High-throughput detection of neutralizing antibodies against SARS-CoV-2 presents a valuable tool for vaccine trials or investigations of population immunity. We evaluate the performance of the . Neutralizing antibodies are critical for conferring immunity against SARS-CoV-2. Here, Dahn et al. report a PCR assay termed SONIA (Split-Oligonucleotide Neighboring Inhibition Assay) for .Serial dilutions of Anti-SARS-CoV-2 Neutralizing Antibody (Catalog# 40150-D002) was detected by SARS-CoV-2 (2019-nCoV) Inhibitor Screening ELISA Kit (Catalog# KIT001). The IC50 (WT) is typically 3.9 nM. Please Note: Optimal .
This product is used for in vitro quantitative detection of anti-SARS-CoV-2 IgG antibodies in mouse serum, plasma and other culture samples. It can help assess the amount of IgG antibodies produced by mouse after immunization or mouse IgG antibodies expressed in cell culture.
The neutralizing antibody titer of convalescent COVID-19 patient plasma was detected by a pseudo virus neutralization assay as described previously [14], [15].In brief, the serial dilutions of the test samples (six dilutions in a 3-fold step-wise manner) were incubated with pseudo virus for one hour at 37 °C, together with the virus control and cell control wells.Figure Legend Snippet: Persistence of maternal milk-derived SARS-CoV-2 antibodies in infant saliva after breastfeeding Milk and saliva samples were collected from mother and infants that were vaccinated with 2 doses of mRNA-based vaccine. (A–D) Two-tailed Spearman correlation was used to correlate milk and maternal saliva anti-Spike IgA levels (A) and IgG (B).
how to test a refractometer
The neutralization activity is measured by microneutralization assay in vitro. The virus microneutralizaiton (MN) test was performed on 293T-ACE2 cells infected with SARS-CoV-2 Spike Pseudovirus (WT, Cat# PSV001), or SARS-CoV-2 Spike Omicron (B.1.1.529) Pseudovirus (Cat# PSV016), or SARS-CoV-2 Delta (B.1.617.2) variant Spike Pseudovirus (Cat# PSV011), .SARS-CoV-2 Spike RBD ELISA Kit (DEIASL411), manufactured by Creative Diagnostics with high sensitivity and reproducibility, providing efficient and accurate result for lab research. . Human Anti-SARS-CoV-2 S1 Monoclonal antibody, clone BIB112 (CABT-CS031) . Mouse Anti-SARS-CoV-2 Spike Neutralizing Monoclonal antibody, clone NN54 (CABT-CS064)
The positive control in B, C is AS35, an Anti-SARS-CoV-2 Spike RBD Neutralizing Antibody, Human IgG1, while in (D), it is the S1N-M122, a known anti-Spike RBD Neutralizing Antibody, Chimeric mAb . The gold standard for diagnosing COVID-19 in the acute phase is RT-qPCR. However, this molecular technique can yield false-negative results when nasopharyngeal swab collection is not conducted during viremia. To mitigate this challenge, the enzyme-linked immunosorbent assay (ELISA) identifies anti-SARS-CoV-2 IgM antibodies in the initial weeks . a Characterization of SARS-CoV-2 S protein-specific antibodies. Upper panels: binding of antibodies to the full-length S protein, S1 protein, and S2 protein was evaluated by ELISA (in duplicates .
Development and production of a SARS-CoV-2 pseudotyped lentivirus. To develop a BSL-2-ready assay to investigate neutralizing antibodies to SARS-CoV-2 in Mexico, we first produced SARS-CoV-2 S . The SARS-CoV-2 pandemic poses the greatest global public health challenge in a century. Neutralizing antibody is a correlate of protection and data on kinetics of virus neutralizing antibody .SARS-CoV-2 Rapid Antigen Test 2.0 Nasal The SARS‑CoV‑2 Rapid Antigen Test 2.0 Nasal is a rapid chromatographic immunoassay for the qualitative detection of SARS‑CoV‑2 nucleocapsid antigen present in human nasal samples. This test is intended as an aid in the diagnosis of SARS‑CoV‑2 infection in individuals with or without symptoms consistent with COVID‑19.This .
Sercon Instruments & Applications
anti-sars-cov-2 neutralizing antibody elisa kit|SARS